


转自:康龙化成综合色站
Photo-inducedDecarboxylativeC-SBondFormationtoAccessStericallyHinderedUnsymmetricS-alkylThiosulfonatesandSS-alkylThiosulfonates
YuGuo1,GuotaoLin1,MengjieZhang1,JianXu1&QiulingSong1,2,3
1InstituteofNextGenerationMatterTransformation,CollegeofMaterialSciencesEngineering,HuaqiaoUniversity,Xiamen361021Fujian,China.
2KeyLaboratoryofMoleculeSynthesisandFunctionDiscovery,FujianProvinceUniversity,CollegeofChemistryatFuzhouUniversity,Fuzhou350108,China.
3SchoolofChemistryandChemicalEngineering,HenanNormalUniversity,Xinxiang453007Henan,China.
—Nat.Commun.,2024,doi:10.1038/s41467-024-51334-5
RecommendedbyDepeiMeng_MC4

ABSTRACT:Duetothehighreactivityandversatilityofbenzenesulfonothioates,significantadvancementshavebeenmadeinconstructingC-Sbonds.However,therearecertainlimitationsinthesynthesisofS-thiosulfonatesandSS-thiosulfonates,especiallywhendealingwithsubstantialsterichindrance综合色站,whichposesasignificantchallenge.Herein,wepresentaninnovativeapproachforassemblingunsymmetricS-thiosulfonatesandunsymmetricSS-thiosulfonatesthroughtheintegrationofdualcopper/photoredoxcatalysis.Moreover,wealsorealizedtheone-potstrategybydirectlyusingcarboxylicacidsasrawmaterialsbyin-situactivationofthemtoaccessS-thiosulfonatesandSS-thiosulfonateswithoutfurtherpurificationandpresynthesisofNHPIesters.Theenvisagedsynthesisandutilizationofthesereagentsarepoisedtopioneeraninnovativepathwayforfabricatingaversatilespectrumofmono-,di-,andpolysulfidecompounds.Furthermore,theyintroduceaclassofpotentsulfenylatingreagents,empoweringthesynthesisofintricateunsymmetricaldisulfidesthatwerepreviouslychallengingtoaccess.

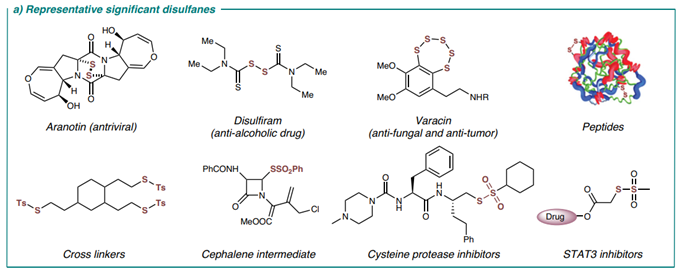
Representativesignificantdisulfanes
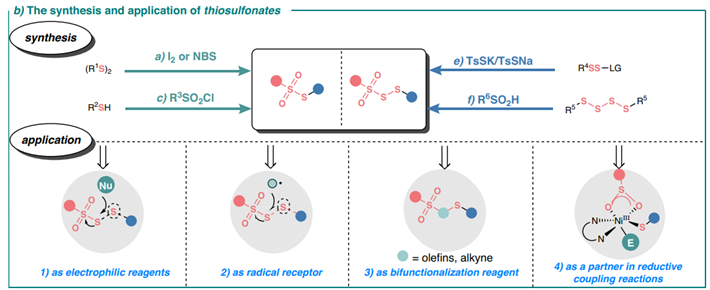
Thegenerationandapplicationofthiosulfonates
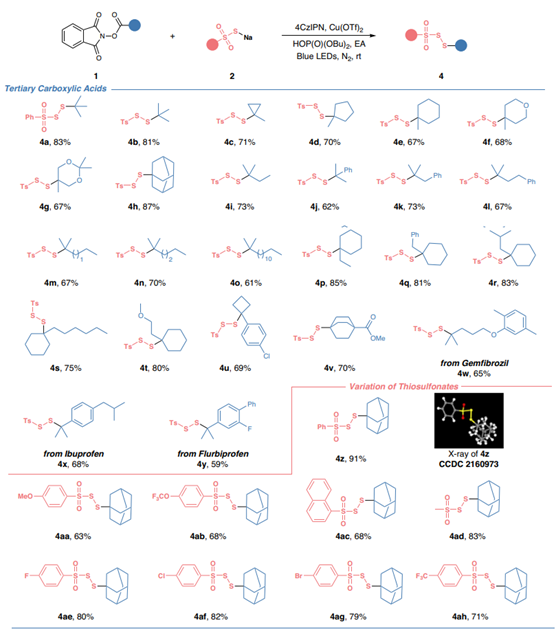
Fromsodiumsulfitetothiosulfonatesandtrisulfidedioxidesviaphotocatalysis(thiswork)

Selectedsubstrates

Proposedmechanism

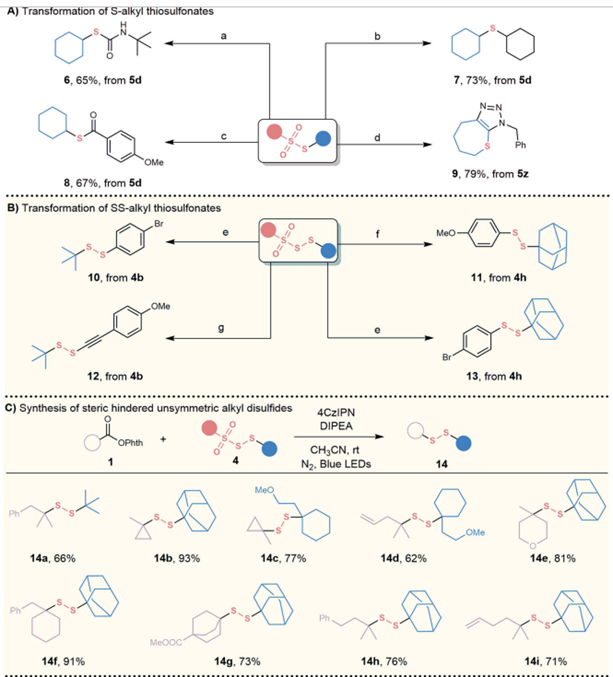
Syntheticapplications
SummaryandComments
Insummary,Prof.QiulingSongreportedavisiblelightinduceddecarboxylativethiosulfonylationreaction.Thismethodisstraightforward,withoutrequiringadditionaloxidantsorreductants,andutilizesreadilyavailableandinexpensivestartingmaterials,thusprovidingarobustpathwayforthesynthesisofunsymmetricS-thiosulfonatesandSS-thiosulfonates,particularlywithsignificantsterichindrance.Thisapproachholdsconsiderablepromiseforbroadapplicationsinthiosulfonylation.Throughsubsequenttransformations,wesynthesizedmono-thiol,di-thiol,andunsymmetricaliphaticdisulfides.
总的来说,福州大学宋秋玲教讲课题组报谈了一种可见光指令的脱羧硫磺酰化响应。该局势阳春白雪,不需要独特的氧化剂或规复剂,提供了一条隆重的,从低价易得的肇始材料启航,合成包括非对称的、具有显著空间位阻的S-硫代磺酸盐和SS-硫代磺酸盐的阶梯。这种局势在硫磺酰化中具有广袤的期骗远景。通事后续的调遣,也不错由此启航取得单硫醇、二硫醇和分辩称脂肪族二硫化物。
(转自:康龙化成)综合色站